Unicamp Professor Contributes to Understanding Schizophrenia and its Relationship with Cerebral Vascularization Changes
Professor and researcher Daniel Martins-de-Souza, from the Institute of Biology at the State University of Campinas (Unicamp), has been awarded the Basic Research Award by the Schizophrenia International Research Society (SIRS). This recognition, bestowed in 2023, marks the culmination of years of dedicated basic research and, notably, significant discoveries in the field of schizophrenia.
Martins-de-Souza, a biologist with a doctorate in biochemistry from Unicamp, has brought to Brazil the knowledge he gained through international research internships. He established the Neuroproteomics Laboratory at Unicamp, where he has distinguished himself through contributions to understanding the molecular structure of schizophrenia. His journey from the Max Planck Institute of Psychiatry to global recognition reflects a tireless effort to unravel the mysteries of the human brain.
The Study
The research that garnered such acclaim, published in the journal Molecular Psychiatry, has deepened our understanding of the complex relationship between schizophrenia and alterations in cerebral vascularization. In collaboration with researchers from the D’Or Institute for Research and Education (Idor) and the Federal University of Rio de Janeiro (UFRJ), the study revealed that astrocytes, cells crucial for maintaining neurons, play a fundamental role in this severe and multifactorial mental disorder.
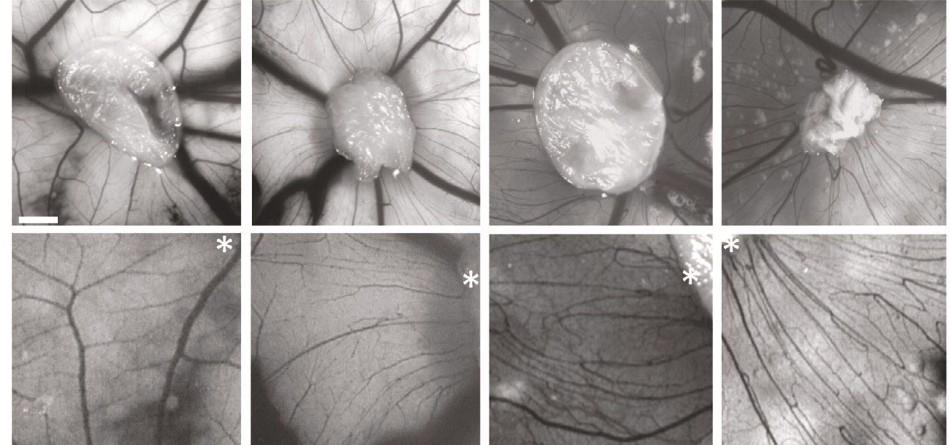
By reprogramming epithelial cells from schizophrenia patients to a pluripotent state, the study team enabled their differentiation into neural stem cells, paving the way for proteomic analysis. Examination of the proteins expressed in the astrocytes of these patients unveiled immune changes and an angiogenic effect on cerebral vasculature.
In a collaborative effort, the researchers also conducted functional assays using a vascular model based on chicken egg membranes, known as CAM. This method provided crucial insights into how substances secreted by patients’ astrocytes impact cerebral vasculature.
“Previous studies had already suggested that both molecular and functional abnormalities of astrocytes could be involved in the pathogenesis of schizophrenia. In our work, we confirmed this relationship through studies with induced pluripotent stem cells. Without this technique, it would have been impossible to study astrocytes in the manner we did,” explained Martins-de-Souza in an interview with Agência Fapesp.
Juliana Minardi Nascimento, the first author of the article, added, “Patient-derived astrocytes showed not only changes in vascularization but also a chronic profile of inflammation.” These findings not only unveil new therapeutic targets for schizophrenia but also illuminate the significance of neurodevelopment from the womb.
“It’s possible that during brain maturation, phenomena like those found in our study occur: systematically altered vascularization leading to the malformation of brain circuits that can trigger schizophrenia in adulthood,” explained Juliana.
The study also emphasized neurological diseases, underscoring the relevance of astrocytes. “The role of glial cells, such as astrocytes, not only in schizophrenia but also in neurological diseases in general, has been a recent discovery, as there was previously a very neuron-centric view of investigating their role. This could be a way to expand our vision and understanding of the disease,” assessed Martins-de-Souza.
For more information about the research, visit the link: https://www.nature.com/articles/s41380-022-01830-1
Research source: FAPESP Magazine.